Pfizer Recalls Blood Pressure Medication Due to Increased Risk of Cancer
Images: Pfizer, FDA
Pfizer recalled one of its hypertension-treating drugs after it was found to have an increased presence of an impurity that can cause cancer.
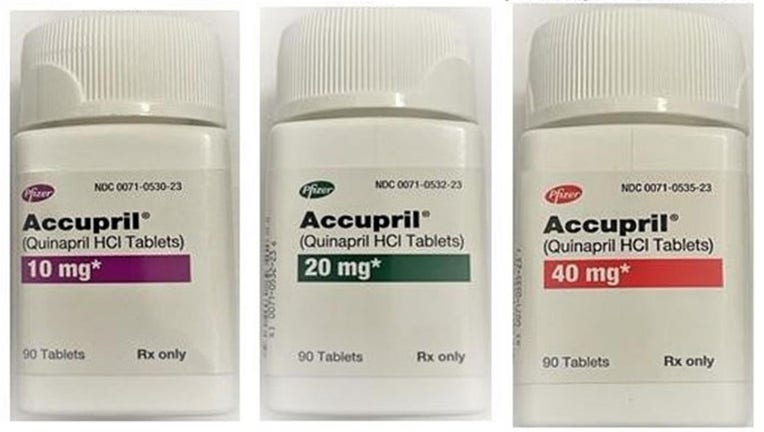
Five lots of Accupril (Quinapril HCl) tablets have been recalled due to a presence of a nitrosamine above the acceptable daily intake level. Increased intake of nitrosamines can increase the risk of cancer over long periods of time.
Those currently taking the affected Accupril tablets are not at any immediate risk and Pfizer is not aware of reports of adverse events related to the recall.
The product lots were distributed nationwide to wholesalers and distributors in the United States and Puerto Rico from December 2019 to April 2022.
The included tablets in the recall all come in 90 count bottles with strengths of 10 mg, 20 mg and 40 mg. You can see the exact NDC and lot numbers included here.
RELATED: Best Buy Insignia air fryer products recalled after reports of fire, burn hazard
Patients who are taking this product should consult with their healthcare provider or pharmacy to determine if they have the recalled product.
Patients with the affected product should contact Sedgwick at 888-345-0481 (Mon.-Fri. 8:00 am – 5:00 pm ET) for instructions on how to return their product and obtain reimbursement for their cost.
According to the U.S. Food and Drug Administration, nitrosamines are common in water and some foods, including cured and grilled meats, dairy products and vegetables. Everyone is exposed to some level of nitrosamines.
Advertisement
This story was reported from Detroit.
" Conservative News Daily does not always share or support the views and opinions expressed here; they are just those of the writer."





Now loading...